Why a Positive D-Dimer Is Not Always a Blood Clot
Here’s why you shouldn’t panic if you learn an elderly loved-one’s D-dimer test came back positive and indicates a blood clot.
The D-dimer blood test is to screen for a blood clot, and doctors routinely order this test when a patient presents to an ER with symptoms that a blood clot in the lung can cause. I learned this firsthand with my mother’s ER visits.
A D-dimer result is more likely to be a false-positive in a person over age 50.
That’s potentially a lot of CT scans (the standard follow-up to a positive D-dimer) and hence, a lot of unneeded radiation, for all those false-positive results.
What is a pulmonary embolism?
“A pulmonary embolism occurs when an artery in the lungs becomes blocked by something that travels through the circulatory system and lodges in the lung,” explains Christopher J. Hanifin, PA-C, who was previously a physician assistant in open heart surgery with Cardiothoracic Surgery of South Bend in South Bend, IN.
“The most common culprit is a blood clot that forms in a large vein in the leg – a deep vein thrombosis – and then breaks loose and is carried to the lungs,” continues Hanifin.
“Depending upon the amount of lung involved, this is usually associated with some degree of shortness of breath.
“PE is also commonly associated with chest pain, which can be vague and difficult to distinguish from other causes of chest pain.”

Shutterstock/michaelheim
Even if you go to the ER with ONLY chest pain, you’ll get a D-dimer test — which is done from a blood sample.
It makes sense that older age is included in the screening, as was in a study that appears in the March 19, 2014 issue of JAMA.
Age of the Patient
The report in JAMA says that using a person’s age to raise up the threshold for an abnormal D-dimer result appeared to be safe, and it led to fewer healthy people getting a diagnosis of a pulmonary embolus.
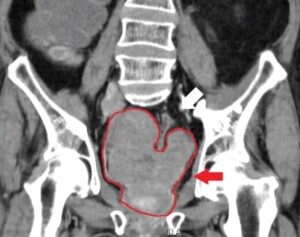
A blood clot causes a breakdown product called D-dimer.
A negative blood result means you don’t have a pulmonary embolism. But with increasing age comes an increased likelihood of a positive result, even if there are no blood clots.
This makes the test less reliable for elderly patients when compared to younger.
Dr. Marc Righini’s study redefined the test value by multiplying the participant’s age by 10 if the patient was at least 50, and using this numerical result with the blood test result.
The team was able to safely exclude a pulmonary embolus diagnosis in elderly patients who were suspected of having a PE.
Over 3,300 participants with suspected PE were involved.
When a patient’s age with a multiplication by 10 was factored into the blood test result, the PE diagnosis was safely excluded.
Some years ago while at the health club, minutes before I was scheduled to train my next client, I called my father to see how my mother was doing, as he had taken her to the emergency room because she was having shooting pains in her upper abdomen.
He told me that the doctor suspected a blood clot in her lung.
This scared the willies out of me and I had difficulty pretending everything was alright while I trained my bubbly client.
“A laboratory test – the D-dimer – can also suggest the presence of a clot in the body, but this test becomes less reliable in older patients,” says Hanifin.
Too bad I hadn’t known, at the time, that being over age 65, in and of itself, can yield a positive D-dimer result in the absence of a blood clot.
Turned out my mother’s lungs were just fine.
Several years later, my mother would end up having at least three more emergency room D-dimer tests, all coming back positive! (Again, lungs just fine each time.)
The second time my mother’s D-dimer was positive, I didn’t have the fear that I’d had several years prior at the gym.
That’s because I knew that it would probably be another false-positive (and it was).
The third time the D-dimer was positive? I thought, “Here we go again, another CT scan and unnecessary radiation.”
Hanifin explains, “A patient presenting with chest pain or shortness of breath with risk factors for PE should generally have a CT scan performed.
“A CT scan can usually identify the presence and extent of any clots fairly reliably.
“A further benefit of the CT scan is that it can often identify other pathology in the event a PE is not the cause of the symptoms.”
The fourth time the D-dimer was positive, my mother was deemed unsuitable for the CT scan due to mild kidney dysfunction, so she was advised to have a “VQ” scan of her lungs.
Chest pain and difficulty breathing can have many causes.
My mother’s fourth D-dimer test was ordered because she complained of trouble breathing (which later was determined by a pulmonologist to be from panic attacks).
 Christopher J. Hanifin, PA-C, is currently Department Chair and Assistant Professor, Department of Physician Assistant, Seton Hall University,
Christopher J. Hanifin, PA-C, is currently Department Chair and Assistant Professor, Department of Physician Assistant, Seton Hall University,
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image source: vecteezy.com
Source:
sciencedaily.com/releases/2014/03/140318162919.htm
How Often Should a Small Abdominal Aortic Aneurysm Be Followed?
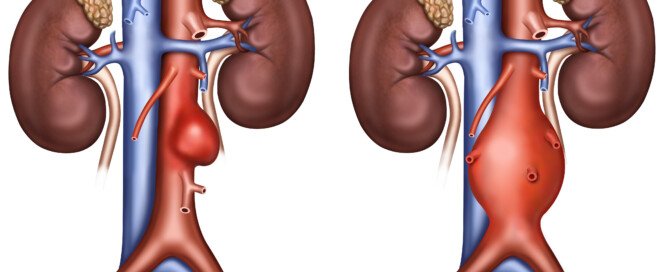
Your doctor will want your small abdominal aortic aneurysm to be followed with a watchful-waiting approach: serial imaging to see if the bulge is getting bigger.
This means imaging at fixed intervals to track any increase in size of the vessel dilation.
But how often should this occur? This depends on the size of the bulge and its growth rate over time.
The smallest abdominal aortic aneurysms often do not get significantly bigger over a course of many years.
But this doesn’t mean a small AAA shouldn’t be imaged with regular intervals of time in between.
Serial imaging is crucial. The survival rate of an abdominal aortic aneurysm rupture is about 20 percent.
Risk Factors
- Smoking
- High blood pressure
- Family history
- Being male and over 70
- Women are by no means immune to an abdomincal aortic aneurysm, and a large study showed that for women, high blood pressure was the leading risk factor,
Though most of these bulges grow at a slow rate, the rate varies from one patient to the next.
Though there’s no one-size-fits-all standard for time intervals between surveillance, there are general guidelines.
Time Intervals for Following an Abdominal Aortic Aneurysm
“Abdominal aortic aneurysms (AAA), defined as ≥ 1.5x the patient’s normal abdominal aortic diameter, should begin to be followed once they have reached 2.5 cm in diameter,” says Brett Mollard, MD, a board certified diagnostic radiologist who specializes in abdominal imaging and nuclear medicine.
“Recommended follow-up frequency is dependent on aneurysm size, with larger aneurysms requiring more frequent follow-up, as they typically have faster rates of growth and higher rates of rupture compared to smaller aneurysms.
“For example, aneurysms in the 2.5-2.9 cm range should be followed at either five year (Journal of the American College of Radiology) or 10 year intervals (The Society for Vascular Surgery); while aneurysms in the 4.0-4.4 cm range should be followed in one year intervals; and aneurysms greater than 5.5 cm in diameter or rapidly enlarging (≥ 5 mm in six months) should typically be treated.”
Conclusions
Cost effectiveness of following an abdominal aortic aneurysm needs to be assessed, as well as different types of surveillance policies.
Reducing imaging frequency would cut surveillance costs, yet also increase patient anxiety and rupture rates. In turn, costs related to emergency surgery would rise.
 Brett Mollard, MD, completed his residency in diagnostic radiology and nuclear medicine at the University of Michigan where he served as Chief Resident. He subsequently completed a fellowship in abdominal imaging at the University of California, San Francisco (UCSF). He currently works in private practice.
Brett Mollard, MD, completed his residency in diagnostic radiology and nuclear medicine at the University of Michigan where he served as Chief Resident. He subsequently completed a fellowship in abdominal imaging at the University of California, San Francisco (UCSF). He currently works in private practice.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image: BruceBlaus
Source: jamanetwork.com/journals/jama/fullarticle/1656254
Endovascular Aneurysm Repair vs. Open Surgery Survival Rates
How much better is the survival rate of an endovascular repair of an abdominal aortic aneurysm when compared to open surgery?
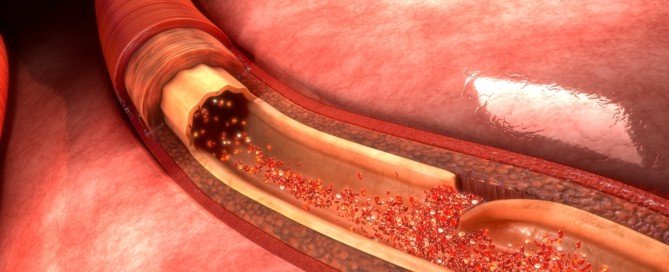
An endovascular repair of an AAA isn’t as complicated when compared to the more invasive, open surgical treatment.
On the other hand, stented grafts can fail, and this procedure requires lifelong CT scans (radiation) for monitoring.
A Johns Hopkins professor has determined that the four-year survival rate between both repair procedures for abdominal aortic aneurysms is similar.
This means that elderly patients, who might have been considered too frail to undergo the invasive open repair, didn’t fare better with the endovascular version – when four-year survival rate was considered.
This is very compelling information for an elderly person who’s afraid to have the open procedure.
No Survival Rate Advantage
For patients over age 70, this study showed that the minimally invasive endovascular grafting did not result in a higher survival rate.
Study leader Julie A. Freischlag, MD, points out in the paper that it’s questionable that very old patients should have any repair to their abdominal aortic aneurysm.
The repairs did not prolong life because these elderly patients were “dying of the diseases of old age,” states the paper.
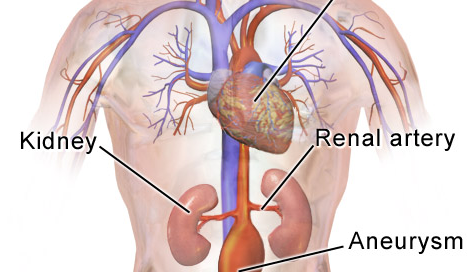
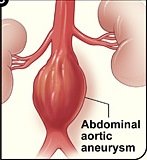
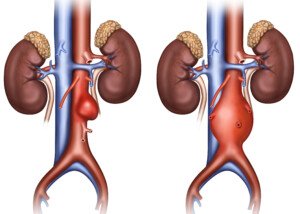
What is an abdominal aortic aneurysm?
An aneurysm is when a portion of a blood vessel wall becomes weak, causing it to swell, dilate or “balloon.”
Think of a balloon filling with air; as it enlarges, its “walls” become thinner and more prone to rupturing.
An AAA can rupture if it gets big enough, causing fatal internal bleeding. They typically cause no symptoms.
People usually learn they have an AAA when it’s accidentally discovered upon imaging for an unrelated concern.
The Johns Hopkins study concluded in 2012, which means that consideration of survival rates for open surgery vs. endovascular repair may be outdated, as advances in repair techniques are always underway.
So as of 2019, I asked Michael Fiocco, MD, about this.
“EVAR has a lower mortality rate than open surgery at 30 days — but the mortality rates converge at three to four years, due to the need for additional procedures, graft migration and potential aneurysm rupture due to migration in the EVAR group,” explains Dr. Fiocco, Chief of Open Heart Surgery at Union Memorial Hospital in Baltimore, Maryland, one of the nation’s top 50 heart hospitals.
This may make you wonder if younger patients, who in general are in better shape than those over 70, might be better candidates for open surgery.
“Younger healthier patients may be better served with open repair, but many factors are involved,” says Dr. Fiocco.
“Youth may not always convey health, and anatomy is just as important. If the aneurysm extends above the renal arteries, or down into the iliac arteries, this can be very challenging to overcome with EVAR, although techniques and equipment continue to improve.”
For endovascular repair the patient must have the appropriate anatomy to “land” the graft.
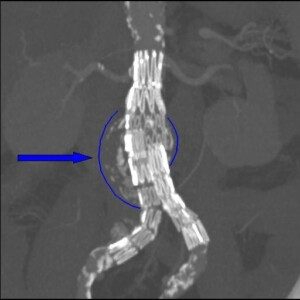
Stent graft on an abdominal aortic aneurysm. Dr Haudebourg, Public domain, via Wikimedia Commons
As mentioned previously, EVAR requires lifelong CT monitoring — every six months — to check for graft shifting (migration).
An intriguing detail in the Johns Hopkins study, which involved 881 patients with an average age of 70, is that the only post-repair aneurysm ruptures occurred in the endovascular group who did not get their regular CT scan follow-ups.

Dr. Fiocco specializes in treating artery disease, valvular disease and aortic aneurysm. His heart care expertise has earned him recognition by Baltimore Magazine as a Top Doctor in 2010, 2011, 2013, 2016 and 2017.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Source: hopkinsmedicine.org/news/media/releases/long_term_survival_rates_after_less_invasive_repair_of_abdominal_aneurysm_the_same_as_with_open_procedure
Best Time to Have Aortic Dissection Surgical Repair

An aortic dissection can kill you in two minutes; certainly there has to be a best time to suffer this life-threatening rupture of the heart’s great vessel.
Unfortunately, you don’t get to decide when an aortic aneurysm starts rupturing.
A study has linked specific time periods with increased survival rates following repair of aortic dissection, and with reduced hospital stays following the procedure.
A report in the online Interactive Cardiovascular and Thoracic Surgery states that when repair of an acute aortic dissection was performed during the period of a waning full moon, odds of death were reduced.
When the surgery was performed during a full moon, hospital stay was reduced. The study was done at Rhode Island Hospital and the paper published in 2013.
“We focused the study on patients having aortic dissection,” says Frank Sellke, MD, senior author, in the paper, “and found that the odds of dying following this procedure were greatly reduced during the waning full moon.”
Two groups of patients were studied:
1) Patients undergoing repair of an ascending aortic dissection, and
2) Those having an aortic dissection plus either coronary bypass surgery, aortic valve surgery or both.
When the repair of an aortic dissection was done during the full moon phase, patients had a substantially shorter hospital stay when compared to the other two moon phases.
This comes down to full moon cycle (10 days) vs. other moon phases (14 days).
What does the moon have to do with aortic dissection repair outcome?
Aortic dissection. Shutterstock/sciencepics
“Although this study was small, it showed clear advantage to those operated on during the full moon,” says Michael Fiocco, MD, Chief of Open Heart Surgery at Union Memorial Hospital in Baltimore, Maryland, one of the nation’s top 50 heart hospitals.
“Is the moon affecting the patient? The surgeon?
No one really knows, but I don’t discount any of these reports.
“There have been for years doctors who swear that the ER is always busier during the full moon; all the crazies come out during the full moon, etc.
“Unfortunately, dissection of the ascending aorta has a mortality of 85-90% within the first 24 hours of diagnosis if left untreated, so you are stuck having surgery during whatever moon phase you are in at diagnosis.”

Dr. Fiocco specializes in treating artery disease, valvular disease and aortic aneurysm. His heart care expertise has earned him recognition by Baltimore Magazine as a Top Doctor in 2010, 2011, 2013, 2016 and 2017.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image: ©Lorra Garrick
Sources:
mayoclinic.com/health/aortic-dissection/DS00605
sciencedaily.com/releases/2013/07/130715141816.htm
Abdominal Aortic Aneurysm Near Renal Artery: Endovascular Repair
Innovative technology makes endovascular repair possible for an abdominal aortic aneurysm when it’s close to the renal arteries.
Have you been told you can’t have endovascular repair of your abdominal aortic aneurysm because the defect is too close to the renal arteries, and that your only option is the invasive, open surgery?
There’s been a breakthrough in stent-graft “landing,” in that now, the stent-graft can be customized to accommodate the patient’s anatomy.
The projection is that this will eliminate a lot of open surgeries, allowing these patients to undergo endovascular repair of their abdominal aortic aneurysm.
One of the first U.S. hospitals to provide this new technology is Johns Hopkins Hospital.
Historically, the open surgery has been performed in some patients because endovascular repair is not possible; the stent-graft would get in the way of the renal arteries because the abdominal aortic aneurysm is located too close to these vessels.
In stent-grafting (which is done by feeding a catheter through the groin to the abdominal aorta), room on either end of the graft is required to “land” it. This room isn’t there if the aneurysm is close to the renal arteries.
Though the open surgery is cheaper, it’s also much riskier (e.g., higher risk of kidney failure or heart attack) and can require up to eight weeks for recovery.
Prior to this new technology, the grafts used for endovascular repair were pulled off the shelves; not tailor-made for the patient.
“We need at least 5 millimeters to 10 millimeters of length between the renal arteries and the aneurysm in order to secure the stent-graft in place in most patients,” says surgeon James Black, in the report, from Johns Hopkins.
Dr. Black is one of only a few dozen surgeons in the U.S. who’ve been trained to make endovascular repairs of abdominal aortic aneurysms with this new graft technology, which was FDA-approved in April of 2012.
Traditional endovascular grafts are made of polyester fabric that’s encased in a stainless steel scaffold.
The newest development is similar, except that it has fenestrations: two little holes or openings fabricated into the graft, allowing accommodation of the renal arteries. These tiny holes help keep the device in place.
The graft must be “engineered correctly to match the patient’s individual anatomy,” explains Dr. Black.
Pre-surgical planning includes using a CT scan to create a 3-D image and model of a patient’s aorta. The construction of these grafts takes around five weeks.
Dr. Black says that every year, Johns Hopkins performs about 100 open surgeries for abdominal aortic aneurysms in patients who do not qualify (due to location of their aneurysm) for the minimally invasive endovascular repair.
But with the new fenestrated device, says Dr. Black, “We will be able to spare many of those patients a big operation and a long recovery.”
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Top image: Shutterstock/ilusmedical
Source: hopkinsmedicine.org/news/media/releases/customized_device_tailored_to_patients_individual_anatomy_now_used_to_repair_abdominal_aortic_aneurysm_without_surgery__
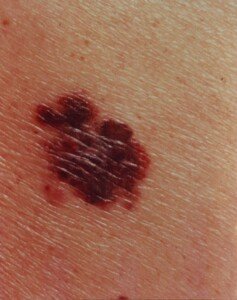
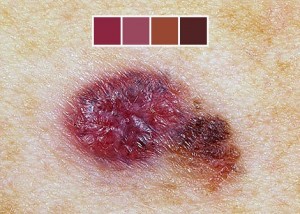
Why Is Melanoma So Hard to Treat?

All advanced cancers are difficult to treat, but one of the most difficult metastases to manage is melanoma skin cancer.
Melanoma is one of the most difficult cancers to treat. However, researchers are closing in on this mystery.
The news is from UC Irvine’s Chao Family Comprehensive Cancer Center; researchers there have figured out a major reason why melanoma is so resistant to chemotherapy treatment and thus exceptionally hard to treat.
While melanoma is highly curable when caught early, says Mayoclinic.com, this cancer yields a very poor prognosis once it spreads, because historically, it evades chemotherapy so well.
The American Cancer Society says that once this disease spreads, the five-year survival rate is 15-20 percent.
Why is melanoma so hard to treat?
Laurence Meyer, MD. cancer.gov
There is a genetic pathway in this cancer that inhibits a cellular mechanism.
This cellular mechanism’s job is to detect damage to DNA. This DNA damage is caused by chemotherapy.
When the cellular mechanism fails, the melanoma cells build up a resistance or tolerance to chemotherapy — which is supposed to kill cancer cells. The researchers targeted this pathway.
The pathway consists of the genes RhoJ and Pak1. UCI dermatologist Dr. Anand Ganesan aims to figure out a way to shut off this pathway.
Dr. Ganesan explains that if the pathway is turned off, “melanoma tumors would suddenly become sensitive to therapies we’ve been using for the last 20 years.”
What exactly is RhoJ and why does this make melanoma so difficult to treat?
It’s a gene that’s involved in the growth of blood vessels. Dr. Ganesan’s team noted that RhoJ does something peculiar when a cancer cell gets hit with chemo drugs.
The gene activates Pak1, another gene. In the study, Pak1 then triggered a molecular chain reaction that suppressed the melanoma cell’s ability to “feel” the damage from the chemo drug.
Think of it this way: The cancer cell gets spanked, but can’t feel the spanking, so the spanking (chemotherapy) does no good. The objective is for the chemo drugs to cause melanoma cancer cells to commit suicide.
Well, they don’t because they don’t sense the drugs’ effect, and as a result, the melanoma cells run amok.
“Being capable of rapid adaptation and change is a hallmark feature of this challenging form of cancer and makes it very difficult to treat,” says Dr. Ganesan.
The team has discovered the why, but has not figured out how to make the spankings hurt (i.e., shut off RhoJ).
However, identifying the “why” is the first step in making melanoma easier to treat.
The researchers are now investigating methods that will suppress these wayward genes so that the cancer cells can sense the DNA damage from chemo.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image: ©Lorra Garrick
Sources:
sciencedaily.com/releases/2012/09/120917132351.htm
mayoclinic.com/health/DiseasesIndex/DiseasesIndex
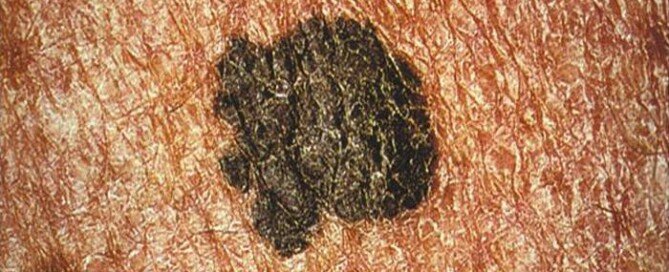
Is There a Way to Detect Melanoma Extremely Early?
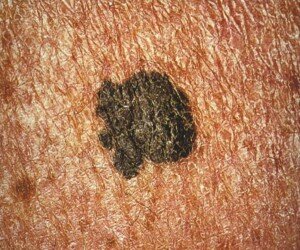
There IS a way to detect and diagnose melanoma when it’s in a very early stage — and hence, highly curable with an excellent 10-year survival rate.
Just how early can melanoma be detected? First of all, some exciting news:
The chief reason why melanoma cells are so resistant to chemo has been discovered by researchers at UC Irvine’s Chao Family Comprehensive Cancer Center.
However, researchers are a long way off from applying this discovery to melanoma treatment. This is why very early detection of melanoma is crucial.
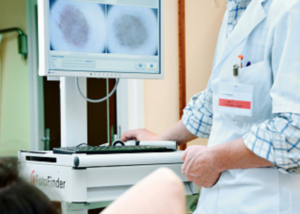
Those who fear this skin cancer and/or are at elevated risk need to know about a technology called serial digital dermoscopy.
Malignant lesions can be missed by the layperson during monthly skin checks and even by a dermatologist, simply because the naked eye cannot always detect changes.
“Monitoring your moles for change or irregular features is one of the best ways to detect melanoma skin cancer early,” says Adam J. Mamelak, MD, a board certified dermatologist and founder of Sanova Dermatology in Austin, TX.
“In the doctor’s office, dermatologists often use handheld microscopes called dermatoscopes to evaluate the features and patterns of a mole or pigmented lesion on the skin.
“Just like the A, B, C, D, E features of atypical moles that we look for with the naked eye, there are a number of characteristics that we look at with the dermatoscope to determine if a spot on the skin is benign or malignant.
“Newer dermatoscopes allow you to connect a phone or camera to the magnifying lens and take pictures of the dermatoscopic image.
“Just as we look for changes in a mole with the naked eye, serial dermatoscopic images can be compared to make sure no irregular features are visible or become visible over time.
“DIgital dermoscopy, just like high resolution digital photography, has become a useful tool in the dermatology clinic.”
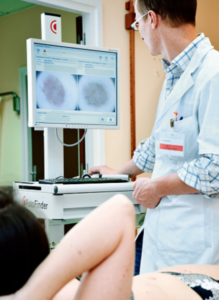
Serial digital dermoscopy
A dermatologist will examine your skin and then select the moles for the serial dermoscopy.
In my case, I requested that the moles on my back — even though typical — get imaged since I can’t easily inspect them.
Selected moles were indicated with a marker so that the nurse knew which ones to image.
Soon after, the dermatologist sat with me at the computer and reviewed the magnified images.
Serial digital dermoscopy involves comparing the images to a database of images of normal and melanoma moles.
Some computer systems will rate the images according to a color spectrum.
Other computer systems don’t do any rating, and instead, dermatologists use their naked eye to inspect the blown-up images on the computer screen for any signs of abnormalities.
When melanoma is caught very early, the 10-year survival rate is 99 percent.
 Dr. Mamelak focuses on the full breadth of dermatologic care, from cosmetic skin solutions to advanced skin cancer removal. He’s founder of the Austin Mohs Surgery Center, which is dedicated to the treatment and management of skin cancer.
Dr. Mamelak focuses on the full breadth of dermatologic care, from cosmetic skin solutions to advanced skin cancer removal. He’s founder of the Austin Mohs Surgery Center, which is dedicated to the treatment and management of skin cancer.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image: CDC, Carl Washington, MD, Emory Univ. School of Medicine, Mona Saraiya, MD, MPH
Source: news.uci.edu/press-releases/uci-researchers-find-cause-of-chemotherapy-resistance-in-melanoma/
Google “Twitching Muscles” and Now You’re Panicking?

Twitching muscles is a symptom of the deadly ALS disease, as this is listed on many medical sites that describe this fatal motor neuron condition.
Many people suffer from a mysterious condition in which they become consumed by intense fear – fear that they will be dead within five years, all because of two things: muscle twitching and the Internet.
The Internet has spawned terror in thousands, maybe tens of thousands of people in America. It begins quite innocently, and here is how:
A person begins to notice some minor, involuntary muscle twitching. They may not think much of it until it starts becoming more frequent, and/or “spreads” all over their body.
When it becomes annoying enough, the person googles cause of muscle twitching, or twitching muscles causes, or something like that.
The person either wants to know what can be causing this nuisance, or might want to see if this is a symptom of multiple sclerosis.
What the person doesn’t realize, is that he or she is in for the terror of his life.
Because when you google these key words, links for ALS come up.
Now, many people don’t know what ALS is, so they’ll click these links and they will soon find out, and their life will change at that moment.
And some other individuals know what ALS is, and when they see the links come up, their heart will start pounding and they’ll break a sweat.
ALS is perhaps the most horrific disease, far more relentless than AIDS, more frightening than cancer, and only Alzheimer’s is worse.
Well, actually, maybe not; at least with Alzheimer’s, there are well-established risk factors, and measures a person can take to really lower the risk.
But sporadic ALS has no known risk factors. The familial (genetic) version accounts for only 5-10 percent of cases.
Furthermore, there are no known measures a person can take to lower risk.
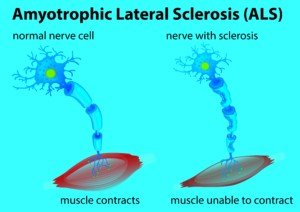
ALS stands for amyotrophic lateral sclerosis, also known as Lou Gehrig’s disease.
When you want to move a muscle, the motor control region of your brain sends an electro-chemical impulse down your spinal cord, to the muscle you want to move, and the muscle contracts.
These impulses travel up to 300 feet per SECOND, which is why the desire to move, and the actual movement, are instantaneous.
- In ALS, the motor neurons in the brain start degenerating. Impulses no longer reach muscles.
- The muscles waste away.
- The person eventually loses the ability to walk and swallow, becomes bedridden and hooked to a respirator.
- Death is 100 percent certain, usually respiratory failure, and comes 2-5 years after diagnosis, though some victims live longer, like renowned phycisist Stephen Hawking.
Muscle twitching is a symptom of ALS. So imagine the sheer terror that a person will feel, when they discover this after googling muscle twitching.
They now believe they might have ALS. Not all googlers will go this route.
But a certain percentage will, even though ALS is at the bottom of the list of causes of muscle twitches.
All the sites generally say the same thing; that muscle twitching, also called fasciculations, is caused primarily by anxiety, stress, dehydration, strenuous exercise, general exercise, deficiency in calcium, magnesium or potassium, medication side effects, unknown causes, viral infections, and a few other miscellaneous causes.
ALS is at the bottom of the lists. But that certain percentage of googlers will fixate on this deadly, incurable disease, and from that moment on, their life will be hell.
Howie Zheng, MD, Neurologist
Howie Zheng, MD, is a general neurologist with The Neurology Center at Mercy Medical Center in Baltimore, Maryland. Dr. Zheng explains:
“I think one of the main things to make people aware of is that fasciculations does not automatically mean you have ALS.
“While the term fasciculations oftentimes leads to a state of panic, it should be noted that there are many different benign conditions that can cause fasciculations. It is a nonspecific term.
“Things such as fatigue, over-activity, anxiety, back problems, nerve compression can all cause fasciculations.
“In the case of ALS, usually fasciculations are accompanied by concomitant muscle weakness, atrophy and deterioration.”
 Dr. Zheng treats patients experiencing fundamental symptoms of neurological conditions including isolated numbness, stiff muscles, weakness, tremors, difficulty swallowing and trouble moving.
Dr. Zheng treats patients experiencing fundamental symptoms of neurological conditions including isolated numbness, stiff muscles, weakness, tremors, difficulty swallowing and trouble moving.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image: Freepik.com, luis_molinero
Perceived Weakness & Muscle Twitching: Reassurance Tips

There IS a way to conquer the perceived weakness and ALS terror that comes from twitching muscles.
If you suffer from perceived weakness from benign fasciculation syndrome (muscle twitching accompanied by other symptoms), then you need a way to overcome this problem.
Perceived weakness can be crippling — from a psychological standpoint.
But if you know how to manage perceived weakness and benign fasciculation syndrome, you can conquer this hell-hole of anxiety and fear.
Benign fasciculation syndrome is a mouthy term that simply means: harmless muscle twitching accompanied by other harmless symptoms.
The word benign means harmless or won’t get worse; fasciculation means twitching muscles; and syndrome means the presence of more than one symptom.
If all of this is benign and harmless, then why would there be overwhelming anxiety?
Well, if you’ve gotten to this article, you already know why.
For other people who clicked on this article out of curiosity over the title, I will explain why perceived weakness is a horrible problem, and how it’s triggered by benign fasciculation syndrome or twitching muscles.
Muscle twitching is so common that tons of people end up doing Internet searches on the keywords of muscle twitching. Invariably, this leads them to ALS sites.
ALS is a terrifying disease, and muscle twitching is one of its symptoms. So is muscle weakness.
A person with a harmless case of twitching muscles may then worry he or she has ALS.
Amyotrophic lateral sclerosis concept
Benign Fasciculation Syndrome — a Very Common Condition
The worry becomes so magnified and intense, that the muscle twitching gets worse, “spreads” to other body parts, and new symptoms appear:
Muscle weakness, muscle cramping and maybe tingling.
However, in benign fasciculation syndrome, muscle cramping, and tingling in the fingertips and toes, are often present.
These may occur for no known reason or may occur from the anxiety of fearing that the muscle twitching is a sign of ALS.
Benign fasciculation syndrome — can appear overnight when fueled by anxiety
When a person learns that in ALS, muscle twitching comes after muscle weakness, they then suddenly start perceiving muscle weakness.
Because this muscle weakness is only imagined, it’s known as perceived weakness.
A person may eventually get past his or her fear of twitching muscles, but then become consumed by recurring bouts of perceived weakness.
The perceived weakness may keep popping up in the same area, or, it may be in different areas each time.
In benign fasciculation syndrome, perceived weakness can be anywhere.
However, a person may suffer ONLY from perceived weakness, with no other physical symptoms. Common sites for perceived weakness are:
– Hands and Fingers g
– Feet
– Tongue
– Arms
– Legs
– Body-Wide
“There is a big difference between ‘perceived’ or subjective muscle weakness and true loss of muscle strength,” says Howie Zheng, MD, a general neurologist with The Neurology Center at Mercy Medical Center in Baltimore, Maryland.
“For instance, if you feel like you’re feeling increasingly tired walking up a hill or up the steps it may not be due to true muscle weakness — as even common things like cardiac and pulmonary issues can give you that impression.
“In a great majority of ALS patients, the muscle becomes weak to the point where it gives out.
“They usually come in with a presentation that the muscle is weak to the point that they are falling while going up the stairs for instance.”
Benign fasciculation syndrome — besides anxiety, can be caused by mineral imbalance, dehydration, calcium deficiency and strenuous exercise.

If you are plagued by this form of hypochondria, keep a journal. It need not be lengthy.
Just record when you get hit with the perceived weakness, and its location.
Over time, you’ll notice that you’ve had PW in the same spot, which comes and goes.
Seeing this pattern will be reassurance that all is well, because in ALS, its muscle weakness is permanent; there is no coming and going! Once it’s there, it’s there to stay.
So if two weeks ago, you finally got over some perceived weakness in your left arm, and now, suddenly, the PW has returned, then remind yourself that for the past two weeks, all has been normal.
In ALS, there is no such thing as a sudden period of normalcy after clinical muscle weakness develops.
Pay attention to how the area feels when you begin fixating on it.
Imprint that feeling in your mind.
Invariably, the PW of that area will eventually disappear. But if it returns, ask yourself, “Isn’t this the exact same feeling I had before?
Yet, nothing came of it; it never got worse, and it disappeared. I’ve HAD this BEFORE. It went away.”
Realizing that it went away will reassure you that it’s only PW, and nothing pathological.
When you can say, “I’ve had this before,” then this will help get you back on track.
 Dr. Zheng treats patients experiencing fundamental symptoms of neurological conditions including isolated numbness, stiff muscles, weakness, tremors, difficulty swallowing and trouble moving.
Dr. Zheng treats patients experiencing fundamental symptoms of neurological conditions including isolated numbness, stiff muscles, weakness, tremors, difficulty swallowing and trouble moving.
 Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
Lorra Garrick has been covering medical, fitness and cybersecurity topics for many years, having written thousands of articles for print magazines and websites, including as a ghostwriter. She’s also a former ACE-certified personal trainer.
.
Top image: ©Lorra Garrick
Should People with Pneumonia Avoid Crowds?

How safe, or harmful, is it for someone with pneumonia to be around crowds, and just what constitutes a “crowd”?
There are two kinds of pneumonia: community and hospital. Community is when the infection is contracted from microbes in the community.
Hospital refers to pneumonia contracted while being a patient at a hospital.
When my mother was diagnosed with pneumonia, the ER doctor told her it was “community,” and that it could have been contracted when she was standing next to a person in a store who coughed or sneezed.
My father thought nothing of wanting to take my mother out to breakfast so that she wouldn’t have to cook while she recovered from pneumonia.
Intuitively, I knew that this was a bad idea, because it’s easy to imagine, even without a microbiologist’s feedback, just how full of germs a restaurant is.
Imagine all the germs on the menus. The menus at restaurants, I have to believe, never get cleaned!
The menus must pass among thousands of hands over the course of one year, and you never know where those hands have been.
The tables get wiped down, but I’ve seen dirty-looking rags being used. I’ve never seen the seats getting wiped down.
And then of course, at a busy time, the restaurant is crowded.
So I talked my parents out of going out for breakfast to avoid any size crowd.
Several days after the pneumonia diagnosis, my mother had a follow-up with her primary care doctor.
The doctor told her to “avoid crowds of three or four people or more.”
















 Dr. Beatty has worked in primary medicine, surgery, accident and emergency, OBGYN, pediatrics and chronic disease management. He is the Doctor of Medicine for
Dr. Beatty has worked in primary medicine, surgery, accident and emergency, OBGYN, pediatrics and chronic disease management. He is the Doctor of Medicine for