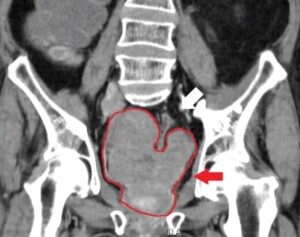
A study shows hope for bladder cancer patients. A little implant has been shown to destroy malignant cells in this organ.
TAR-200 is a tiny, pretzel-shaped device that holds the chemotherapy drug gemcitabine.
It’s placed into the bladder through a catheter, where it slowly releases the drug for three weeks at a time.
A phase 2 clinical (as of 2025) trial just showed some pretty incredible results with this system.
In patients with high-risk non-muscle-invasive bladder cancer — people whose tumors hadn’t responded to previous treatment — TAR-200 wiped out tumors in 82% of them.
Most of the cancers were gone within the first three months.
And almost half of the patients were still cancer-free a year later.
Dr. Sia Daneshmand, a urologic oncologist at Keck Medicine of USC and the study’s lead author, says these patients normally have very few good options.
He called the results the most effective therapy seen so far for this common bladder cancer type.
He also says the trial represents a major breakthrough with real potential to save lives.
So, how does this little device actually work?
TAR-200 sits inside the bladder and slowly infuses gemcitabine directly onto the bladder tissue for weeks.
Before this system, gemcitabine was usually delivered in liquid form and stayed in the bladder for just a few hours.
That short exposure limited how well it could kill cancer cells.
The paper explains the theory behind the new method: The longer the drug stays in contact with the bladder lining, the deeper it can penetrate and the more cancer cells it can destroy.
And based on the trial outcomes, releasing the drug gradually over weeks instead of hours really does seem to make a big difference.
The global study — called SunRISe-1 — was run at 144 sites worldwide, including Keck Hospital of USC.
It enrolled 85 patients with high-risk non-muscle-invasive bladder cancer.
This is the most common bladder cancer type, and it becomes “high risk” when the tumors are more likely to return or spread deeper into the bladder wall.
All the patients had already tried BCG, the standard immunotherapy for this cancer.
But for many people, BCG just doesn’t work, and the cancer comes back.
Until now, the usual backup plan was major surgery to remove the bladder and sometimes nearby organs.
That surgery has big risks and can seriously affect a patient’s quality of life.
Researchers wanted something safer and easier to tolerate.
How the Study Was Done
In the trial, TAR-200 was given every three weeks for six months, then four times a year for two additional years.
Out of the 85 patients, 70 had their tumors completely disappear.
Almost half stayed cancer-free for at least a year.
Side effects were minimal, and overall the treatment was well-tolerated.
Interestingly, combining TAR-200 with another immunotherapy drug called cetrelimab actually worked worse and caused more side effects.
Study participants will continue to be monitored for another year, although enrollment is complete.
This research fits into a bigger trend of using slow-release drug systems that deliver medication directly where it’s needed.
TAR-200 has now been approved by the FDA and is being sold under the name INLEXZO.