UPDATE on the breakthrough copper study that halted ALS progression in mice.
The copper-ATSM research is moving along since early 2016.
Brief Recap of Copper-ATSM ALS Mice Study
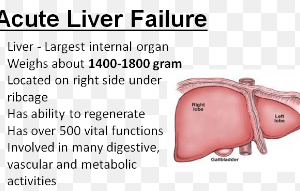
Mitochondria are energy “organs” inside cells. In ALS they are damaged. The copper-ATSM (also known as CuATSM) aids in delivering copper to cells with damaged mitochondria.
In one type of mouse model, ALS progression was halted with CuATSM. These mice lived for over 650 days. Mice that didn’t get the copper-ATSM were dead in two weeks.
When the CuATSM treatment was withdrawn in some of the mice, the ALS symptoms came back within two months.
These mice were dead in a month without a return to treatment. In mice that were re-treated, ALS symptoms again vanished; they lived six to 12 months.
I was in contact with the lead study author, Joseph Beckman, PhD, an experimental biochemist and professor of biochemistry and biophysics at Oregon State University.
That was in 2017. Research is still ongoing as of 2020 (more on that coming).
But first, it’s important that you know how the 2017 interview went.
Dr. Beckman replied via e-mail, “There will be a presentation at the Boston MND meeting in December [2017] on human studies.
“They are only phase I and I cannot say much nor do I know the results.
“The drug seems to be tolerated as far as I can tell. Frustratingly slow progress but actually fast for human trials.”
Key Points as of July 2017: Copper ATSM Treatment for ALS
• The experiment on mice indicates the cuATSM drug “should be safe,” says Dr. Beckman’s report, “but there is a possibility that in humans of significant liver damage or other toxic effects.”
• There’s no proof that cuATSM will work in ALS patients who do not have SOD mutations, but also no evidence it won’t work.
Dr. Beckman’s report explains, “It starts with small doses and will test to see how well is the drug tolerated. The lowest dose was well tolerated and the investigators plan to escalated [sic] to a higher dosage.”
The length of time from human safety testing to approval as treatment is far from short, due to analysis of risks vs. benefits.
ALS Patients Are Dying; Why Not Just Cut to the Chase and Give Them the Drug in Full Dose?
“Probably the most common theme raised by patients and families is what do I have to lose,” says Dr. Beckman’s report.
“The answer unfortunately is a lot. For example, copper is toxic in the brain and the liver.”
Copper-ATSM tightly binds to copper, preventing toxicity – in the lab mice and rats. “We do not know if this will be true in humans in general.”
Furthermore, “It is possible that this drug at the wrong dose would destroy the liver in a day. This would be a slow awful death” without a fast liver transplant.
“The drug might work well in 2-3 patients but then end up killing dozens of patients in a larger scale.”
Many tests must be conducted to avoid these hypothetical but realistic outcomes.
Another so-called holdup to giving the drug in therapeutic doses to ALS patients is the conundrum of how to formulate it (pill? injectable solution? dosing and frequency?).
“These are just a few of the challenges that must be overcome before producing a drug for human use.”
Here is an update in 2021 (no updates are available as of 2023) on the copper research.

 Dr. Beckman’s
Dr. Beckman’s